Knowing what’s really inside our everyday products goes beyond just reading ingredient labels and the packaging. In fact, a Certificate of Analysis (COA) can help to spill the secrets of what makes up our favorite items. What is a Certificate of Analysis? How do you request one from a brand? What should you look for? We’ve got you covered.
Keep reading to learn more about this important quality assurance document!

Note: This article contains affiliate links, meaning In On Around will make a small commission at no additional cost to you. This helps us maintain the site. As always, we value full transparency & only work with brands we love and trust.
Author: In On Around Founder & CEO, Catherine Power.
- Published On: February 19, 2024
- Updated On: April 10, 2024
Summary:
- A Certificate of Analysis can help you evaluate a product’s quality.
- For food products, you can request a COA from the brand to review their heavy metal levels.
Table of Contents
What is a Certificate of Analysis (COA)?
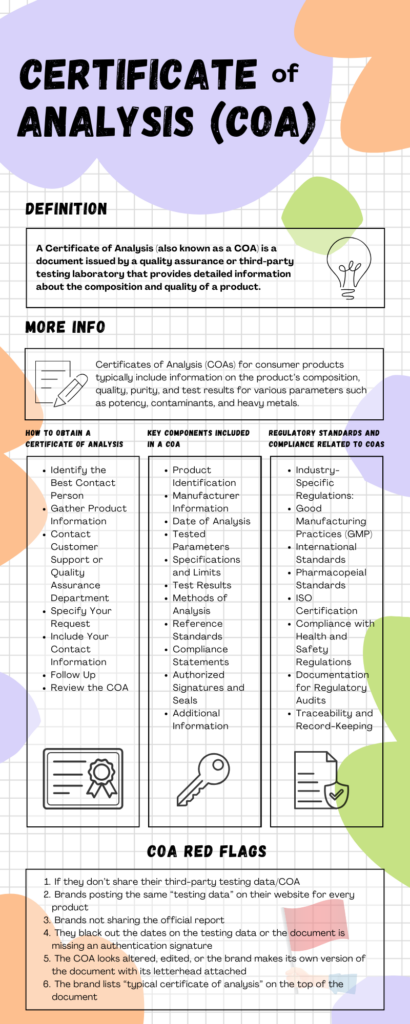
A Certificate of Analysis (also known as a COA) is a document issued by a quality assurance or third-party testing laboratory that provides detailed information about the composition and quality of a product.
It typically includes data on key components, concentrations, purity, heavy metals, bacterial count, and other relevant metrics. It’s used to ensure that the product meets specified standards and regulations (which can vary depending on the product type).
COAs are crucial for verifying the quality and safety of various products, especially in industries such as pharmaceuticals, food, and chemicals.
COA Purpose and Benefits
The Certificate of Analysis (COA) plays a vital role in ensuring product compliance with agreed-upon standards for customers, manufacturers, and suppliers.
From a manufacturing perspective, it is especially essential for importing and exporting food products. It has an even stricter analysis process for pharmaceuticals and chemicals. It is also a critical component in supplier transactions, manufacturing, delivery, and documentation. Companies will oftentimes receive COAs when they buy raw materials from suppliers, so they know if what they’re receiving is pure, safe, and compliant with their requirements.
From a customer/consumer perspective, the COA allows you to review the quality of the final product by manufacturing run. It allows you to better understand the supply chain and what testing the brand is doing on the final product.
If you’re considering purchasing a supplement, it’s always a good idea to reach out to the brand to request a COA.
How to Obtain a Certificate of Analysis
Not all companies will share COA results. In fact, many won’t. Support brands that are proud and transparent with their test results!
To request a Certificate of Analysis (COA) from a brand, follow these steps:
- Identify the Best Contact Person:
- Oftentimes, you need to receive a COA from the brand’s Quality team. Usually, you can contact the brand’s general Customer Service email from their website.
- You can also ask the Customer Service team for a direct email contact within their Quality department. Some brands will share this, others will not. Sometimes Customer Service will send you the COA directly.
- Gather Product Information:
- Collect the specific details about the product, including the product name and any other relevant identifiers like SKU or LOT numbers.
- Typically, you just need the full product name to request a COA.
- Some brands won’t share COAs unless you have a specific LOT number to provide, meaning you would need to purchase a product and share the manufacturing number that’s on the package.
- Contact Customer Support or Quality Assurance Department:
- Reach out to the brand’s customer support or quality assurance department. This can usually be done through the brand’s official website, customer service phone number, or email.
- It’s best to contact them via email so you have a paper trail of how often you have reached out.
- Specify Your Request:
- Clearly state that you are requesting a Certificate of Analysis for a specific product. Request their most recent COA for that product – or their most recent COA for that manufacturing run.
- You don’t need to include why you’re asking – keep the email short and concise.
- Include Your Contact Information:
- Provide your contact information, including your name, email address, and phone number. This ensures that the brand can easily respond to your request.
- Follow Up:
- If you do not receive a response within a reasonable time frame (usually one week), consider following up with the brand to ensure that your request is being addressed.
- If you have followed up with a brand 2-3 times and they have not responded, it’s usually because they don’t want to share the information with you. Try to find a different contact if possible… or assume that they are not being transparent.
- Review the COA:
- Once you receive the COA, carefully review the document to ensure it contains the required information (like heavy metal count, which should detail lead, arsenic, cadmium, and mercury).

Key Components Included in a COA
A Certificate of Analysis (COA) typically includes key components that provide comprehensive information about the quality and composition of a product. The specific components may vary based on the type of product and industry, but common elements found in a COA include:
- Product Identification:
- Name of the product, LOT or batch number, and any other specific identifiers.
- Manufacturer Information:
- Details about the company or entity responsible for manufacturing the product.
- Date of Analysis:
- The date when the analysis of the product was conducted.
- Tested Parameters:
- A list of parameters or characteristics that were tested, such as potency, purity, composition, concentration, and other relevant factors.
- Specifications and Limits:
- The established specifications and acceptable limits for each tested parameter, indicating whether the product meets the required standards.
- Test Results:
- Actual results of the tests performed, including numerical values, percentages, or qualitative assessments.
- Heavy metals, like lead, arsenic, cadmium, and mercury (this is what I love to look at!)
- Methods of Analysis:
- Description of the methods and procedures used to conduct the tests. This ensures transparency and allows for reproducibility.
- Reference Standards:
- Information about any reference standards or materials used in the analysis, ensuring traceability and reliability of the results.
- Compliance Statements:
- Statements indicating whether the product complies with relevant regulations, industry standards, or specific customer requirements.
- Authorized Signatures and Seals:
- Signatures and seals from authorized personnel or laboratories responsible for conducting the analysis.
- Additional Information:
- Any additional information relevant to the product, its handling, storage recommendations, or precautions.
Note that the specific components included in a COA can vary based on the nature of the product, industry regulations, and customer requirements.
COA Red Flags
So, how do you know if a company isn’t being transparent with their test results?
First and foremost, if they don’t share their third-party testing data/COA, then they’re not being transparent. Some companies will claim that their testing data is proprietary – this is simply not true. The level of heavy metals found on the COA is not a “trade secret”, despite what some brands will tell you. This just means that they don’t want to be transparent. If there is confidential information, like addresses or contact information, on the COA, they can redact it (aka black it out) so it’s not public knowledge.
Here are a couple of other red flags to be aware of when requesting Certificates of Analysis:
- Brands posting the same “testing data” on their website, but it’s the same for every product
- This is clearly not true – testing data should be unique and vary slightly by product and manufacturing run. It’s best to see the official test report PDF.
- Brands not sharing the official report – they just post numbers on their website without any proof that those numbers are accurate.
- How are we to know if those numbers are true? We need to see the actual report.
- They black out the dates on the testing data or the document is missing an authentication signature
- Some companies will choose to only share their “best” reports with customers. While this is more transparent than sharing nothing at all, it’s important to see their most recent testing data for manufacturing runs – if you’re able to obtain it.
- The COA looks altered, edited, or the brand makes its own version of the document with its letterhead attached.
- This can be deceptive. If a brand is re-creating the data on a new document and it doesn’t have the official authentication signatures, then it’s a red flag.
- The brand lists “typical certificate of analysis” on the top of the document.
- This means that you likely don’t have the most up-to-date manufacturing run COA.
When requesting documentation from brands, be aware that although some brands are transparent, others may refuse to provide testing data or send documents that do not match your specific request.
Regulatory Standards and Compliance Related to COAs
Ensuring the accuracy and reliability of Certificate of Analysis (COA) documents is paramount in meeting regulatory standards across various industries. Compliance with specific guidelines and regulations guarantees the quality and safety of products. Here are key considerations related to regulatory standards for COAs:
- Industry-Specific Regulations:
- Different industries, such as pharmaceuticals, food and beverages, and chemicals, have specific regulations governing the production and distribution of products. COAs must adhere to these industry-specific guidelines.
- Good Manufacturing Practices (GMP):
- Many industries follow Good Manufacturing Practices to maintain consistent product quality. COAs play a crucial role in demonstrating adherence to GMP, ensuring that products meet the required standards at every stage of production.
- International Standards:
- Products intended for international markets often need to comply with international standards and specifications. COAs facilitate cross-border trade by providing standardized information that aligns with global regulatory requirements.
- Pharmacopeial Standards:
- In the pharmaceutical industry, adherence to pharmacopeial standards, such as those outlined by the United States Pharmacopeia (USP) or the European Pharmacopoeia (Ph. Eur.), is essential.
- ISO Certification:
- ISO certifications, particularly ISO/IEC 17025 for testing laboratories, contribute to the credibility of COAs. Laboratories following ISO standards demonstrate competence and compliance with international norms.
- Compliance with Health and Safety Regulations:
- COAs should address health and safety concerns, providing information on any hazardous substances present in the product. Compliance with regulations such as the Occupational Safety and Health Administration (OSHA) standards is crucial.
- Documentation for Regulatory Audits:
- Regulatory authorities may conduct audits to ensure compliance. A well-documented COA serves as critical evidence during audits, showcasing the product’s conformity to established standards.
- Traceability and Record-Keeping:
- Regulatory compliance often requires traceability and thorough record-keeping. COAs should include details on the traceability of testing methods, reference standards, and the entire testing process.
In conclusion, adherence to regulatory standards and compliance with industry-specific guidelines are imperative for the credibility and acceptance of COAs. Companies must stay informed about evolving regulations to continuously update and enhance their COA practices.

Should You Review COAs Before Buying Products?
This comes down to your personal risk tolerance. Personally, I like to ask for COAs from brands for food products or supplements, especially to see what their heavy metal testing looks like. While this isn’t always possible for every product you ever use, it can be a great practice to ensure that the brands you’re using are low in heavy metals.
If you don’t want to request COAs yourself, don’t worry – that is what In On Around is for! Check out our favorite product picks in our shop and blog posts. If you want us to review a specific product or brand, check out our consulting packages!
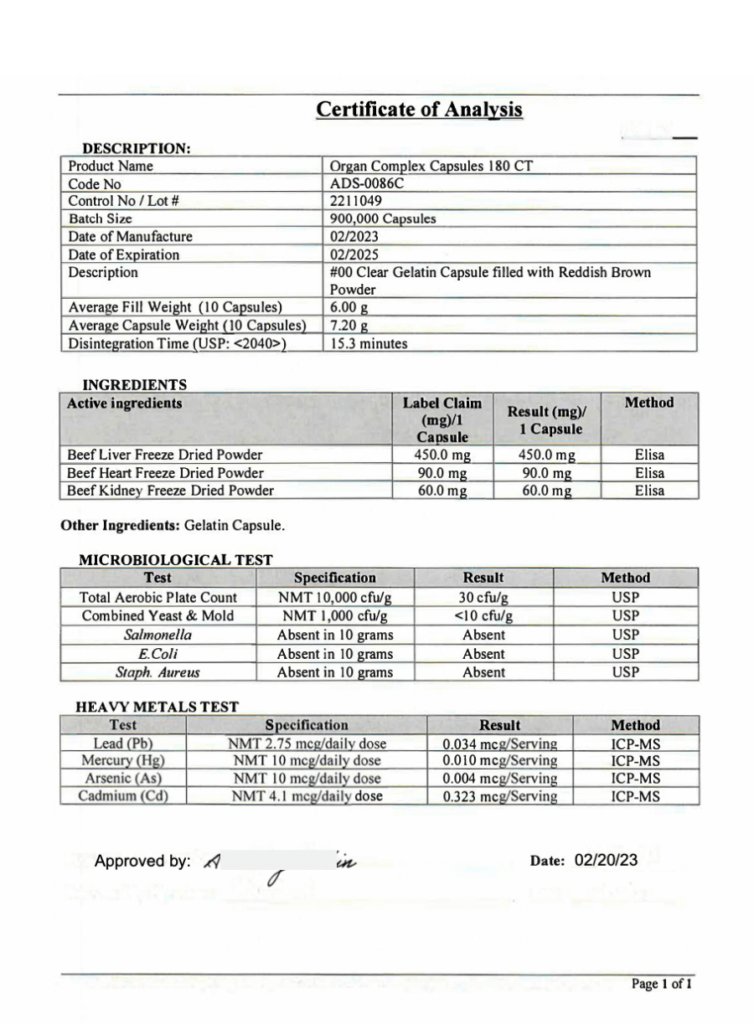
Certificate of Analysis Examples
During my time in the Food & Personal Care industry working for one of the largest Fortune 500 conglomerates, it was my job to review Certificates of Analysis for foreign suppliers. Now, with In On Around, I make it a priority to request COAs from brands, especially for supplements.
Here are some example COAs that we’ve received from brands for your reference. Some come redacted (with black blurred out sections). We redacted some additional information in grey to keep the identities of these brands more confidential.


Final Thoughts - Quality Control Via COA
Certificates of Analysis (COAs) play a crucial role in bringing transparency and informed choices to consumers. It is essential to thoroughly review the test results, including aspects of quality control and analysis, to gain a comprehensive understanding of a product’s purity.
Unless you’re reading quality assurance documents, you don’t get the full picture of how “clean” a product really is. Therefore, by requesting and reading COAs, you can make more informed decisions about the products you’re using daily.
Have you ever requested a COA from a brand?
Let me know your thoughts and key takeaways in the comments below!
xoxo,

Want to read more? Check out our other articles here!
Other references on What is a COA?: DocXellent, Safety Culture, Illumina, LabHut, Smart Food Safe
Copyright In On Around LLC 2024 ©. The statements made on this website have not been evaluated by the FDA (U.S. Food & Drug Administration). They are not intended to diagnose, treat, cure, or prevent any disease. The information provided by this website should not be used as individual medical advice and you should always consult your doctor for individual recommendations and treatment. The information contained in this site is provided on an “as is” basis. Related to this site, there are no guarantees of completeness, accuracy, usefulness, or timeliness. In On Around LLC assumes no responsibility or liability for any errors or omissions in the content of this site.
Frequently Asked Questions – Quality Control via COA
Click on the below FAQs to learn more about: FDA Guidelines, What is COA?, COA certificate sample PDF, Certificate of Quality, Certificate of Analysis Food, Certificate of Analysis for export, and Certificate of analysis online.
No, a COA (Certificate of Analysis) is not the same as a COC (Certificate of Conformity).
Consumers can interpret and understand the test results in a Certificate of Analysis by carefully reviewing parameters such as heavy metal testing, which is critical for assessing product safety. Paying attention to the concentrations of heavy metals reported in the COA helps consumers make informed decisions about the safety and quality of the product.
Reviewing Certificates of Analysis is crucial for consumers as it provides detailed information on product composition and safety. It helps you make informed decisions based on factors like quality, purity, and adherence to standards.
Certificates of Analysis (COAs) for consumer products typically include information on the product’s composition, quality, purity, and test results for various parameters such as potency, contaminants, and heavy metals.








